Abstract
Introduction
Cyclophosphamide (CTX) is a widely used anti-neoplastic, performing as an alkylating agent at high doses and immunomodulatory agent at low doses 1.. Combining CTX with monoclonal antibody (mAb) therapy has proven beneficial in potentiating relapsed and/or refractory multiple myeloma (RRMM) therapies, with daratumumab-directed MM cell death enhanced in the presence of CTX 2,3.. Elotuzumab (ELO), the second mAb approved for treating RRMM, promotes MM cell clearance by enhancing macrophage-mediated phagocytosis and CD16- and SLAMF7-directed NK cell cytotoxicity. ELO has been approved for use alongside dexamethasone and lenalidomide 4 or pomalidomide (POM) 5.. However, potential therapeutic benefits of ELO in combination with immunomodulatory drugs such as CTX and POM have yet to be examined. Our research investigates, the efficacy of combining low-dose CTX, alone or in combination with POM, and ELO in enhancing macrophage and NK cell infiltration and function in the MM tumour microenvironment.
Materials and Methods
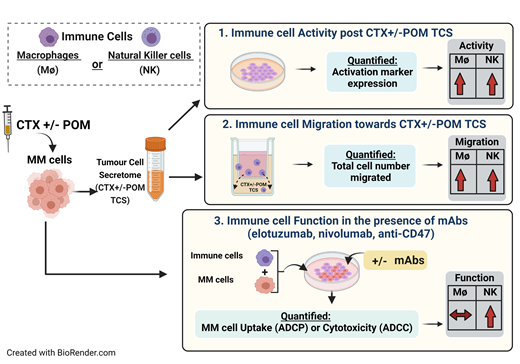
Multiple myeloma cells (MM1S and H929) were treated with low-dose CTX and/or POM for 24hrs, washed to remove residual drug and resuspended in fresh media for tumour cell secretome (TCS) generation. Direct effects of CTX and/or POM on surface expression of checkpoint proteins (PD-1 and CD47) on MM cells was assessed by mean fluorescent intensity (MFI) flow cytometry. CD32/CD64 receptor expression on THP-1 macrophages, NKG2D, CD2, DNAM-1, CD96 and KIR2DL1 receptors on KHYG1 and primary NK cells, were measured using flow cytometry as a measure of activation. Migration of serum-starved, CFSE-labelled macrophages and NK cells towards CTX and/or POM TCS was assessed after 4hrs, with total number of migrated cells quantified using the Accuri flow cytometer. Immune cell function following indirect conditioning of macrophages/NK cells with MM cell TCS was measured by quantifying antibody-directed cellular phagocytosis (ADCP) or antibody-directed cellular cytotoxicity (ADCC), respectively. Conditioned immune cells were co-cultured with MM cells in a 2:1 effector to target ratio for 4hrs in the absence/presence of mAbs (ELO, nivolumab and anti-CD47), after which MM cell clearance was quantified by flow cytometry and presented as relative uptake (ADCP) and cytotoxicity (ADCC). One-way ANOVA statistical analysis was performed, followed by Tukey post hoc tests, with significance recognized at p<0.05.
Results
Direct treatment of MM cells with CTX increased surface expression of immune evading checkpoint proteins PD-1 and CD47 (p<0.05,n=3). POM monotherapy did not alter PD-1/CD47 expression, however dual therapy of CTX and POM supported the CTX-driven effect (p<0.001,n=3). Expression of CD32/CD64 macrophage activation markers was significantly increased on THP-1 cells following CTX-TCS conditioning (p<0.001,n=3). POM altered CD32, but not CD64, however dual treatment with CTX and POM significantly increased expression of both CD32 and CD64 (p<0.001, n=3). Migration of macrophages towards CTX-TCS was enhanced in a dose-dependent manner (p<0.01,n=3). CTX and POM dual therapy supported this CTX driven effect (p<0.001,n=3). Migration trends of both primary and KHYG1 NK cells were also increased towards the secretome from CTX treated MM cells. ADCP and ADCC were increased by CTX alone or in combination with POM (p<0.05, n=3). Effects of CTX on ADCP were not significantly enhanced by ELO, however ELO did significantly augment ADCC by CTX-conditioned primary NK cells (p<0.05,n=3). Given the increased expression of PD-1 and CD47, we investigated if the inclusion of nivolumab and anti-CD47 mAbs potentiated ADCC. Although ADCC was increased in all combinations, there was no significant difference between ELO alone versus ELO in combination with either nivolumab or anti-CD47.
Conclusions
Low-dose CTX and POM potentiated the immunomodulatory effects of ELO, with NK-directed cytotoxicity of MM cells enhanced in the presence of this mAb. Our data therefore indicates that the inclusion of low-dose CTX and or POM in combination with ELO could be a novel immunotherapeutic strategy for treating RRMM.
References
1. Swan et al., Hemasphere. 2020;4(2).
2. Pallasch et al., Cell. 2014; 156(3):590-602.
3. Naicker et al., Oncoimmunology. 2021; 10(1):1859263
4. Dimopoulos et al., Blood Cancer Journal. 2020 10:91
5. Hose et al., Journal of Cancer Research and Clinical Oncology. 2021; 147:205-212
No relevant conflicts of interest to declare.